41 open-label study bias
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,... Open-Label Trial - an overview | ScienceDirect Topics the difference of protective efficacy of ribavirin in humans and in hamsters may have several explanations: (1) the study in humans was not randomized and performed at posteriori, and grouping treated and nontreated patients may have introduced some bias; (2) the dose and mode of infection of niv used for the challenge or the virus replication in …
Investigating Potential Bias in Patient-Reported Outcomes in ... While open-label bias is an important consideration for PRO results, bias related to knowledge of treatment assignment is not unique to PRO and may also affect other common trial outcomes, including progression-free survival 6 and clinician-reported safety data. For instance, the same forces that may lead patients to report fewer or milder ...
Open-label study bias
PDF Clinical Trial Perspective - Open Access Journals hypothetically) the bias would still be present and would still invalidate the results. The ICH E9 guidance dis tinguishes operational and statistical bias; the former refers to bias introduced during the conduct of the trial, whereas the latter refers to bias introduced in the design, analysis and evaluation of the results. It is also A Prospective, Randomized, Open Label Trial of Two Doses of Oral ... None (Open Label) Primary Purpose: Treatment: Official Title: A Prospective, Randomized, Open Label Trial of Two Doses of Oral Betaine in Patients With Non-alcoholic Fatty Liver Disease (NAFLD) and Elevated Alanine Aminotransferase (ALT) Actual Study Start Date : November 12, 2013: Estimated Primary Completion Date : December 30, 2021 Open Label Study | Suggestion Keywords | Top Sites Open-Label Study to Evaluate the Safety, … This is a Phase-1, single center, open label study in approximately 20 adult healthy subjects (Cohort 1 and Cohort 2). Ten subjects will be enrolled in each of the 2 cohorts. The dose of EXPAREL will be determined by the cohort.
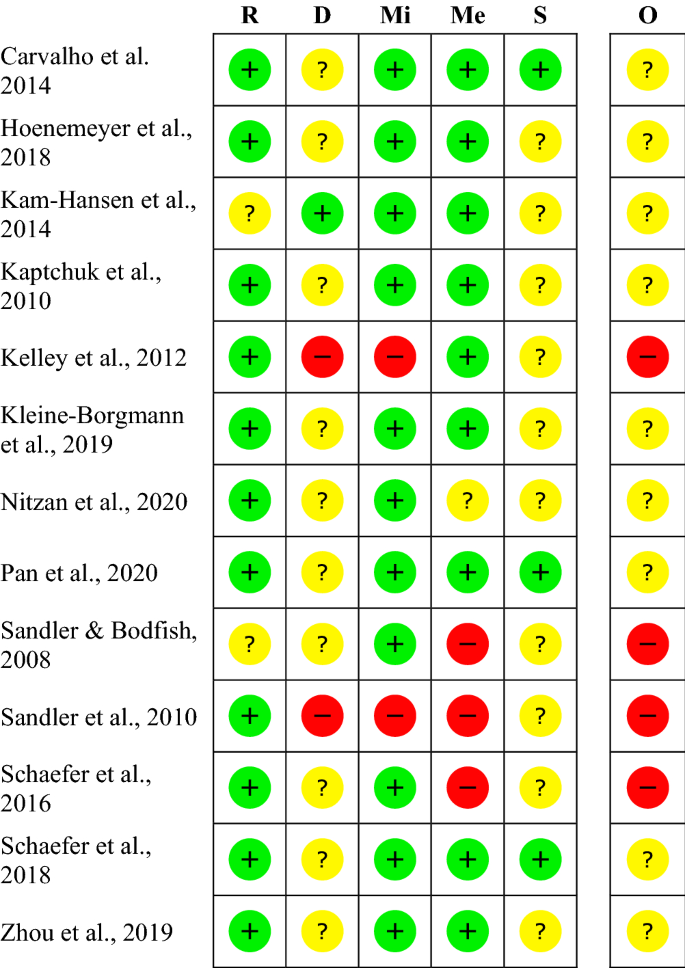
Open-label study bias. PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG Even for open label studies, it's still desirable blind the study sponsor to reduce potential bias due to the sponsor knowing the treatment level aggregated data while the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in ... Investigating the impact of open label design on patient‐reported ... Bias may occur in open‐label trials, as observer bias and disappointment bias. 34 , 35 , 36 , 37 Therefore, according to the FDA, patients may be prone to provide biased reports of their own symptoms if they are aware of the treatment they received and lead to an overestimation of the treatment difference observed between the two treatment arms. Appendix C Risk of Bias Assessment Forms and Summaries There are three categories for describing the overall risk of bias for assessed studies: low risk of bias; moderate risk of bias; and high risk of bias. ... Torres E, et al. Divalproex sodium-ER in outpatients with disruptive behavior disorders: a three month open label study. Child Psychiatry Hum Dev. 2010 Jun; 41 (3):274-84. Open label extension studies and patient selection biases In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%. Conclusions The...
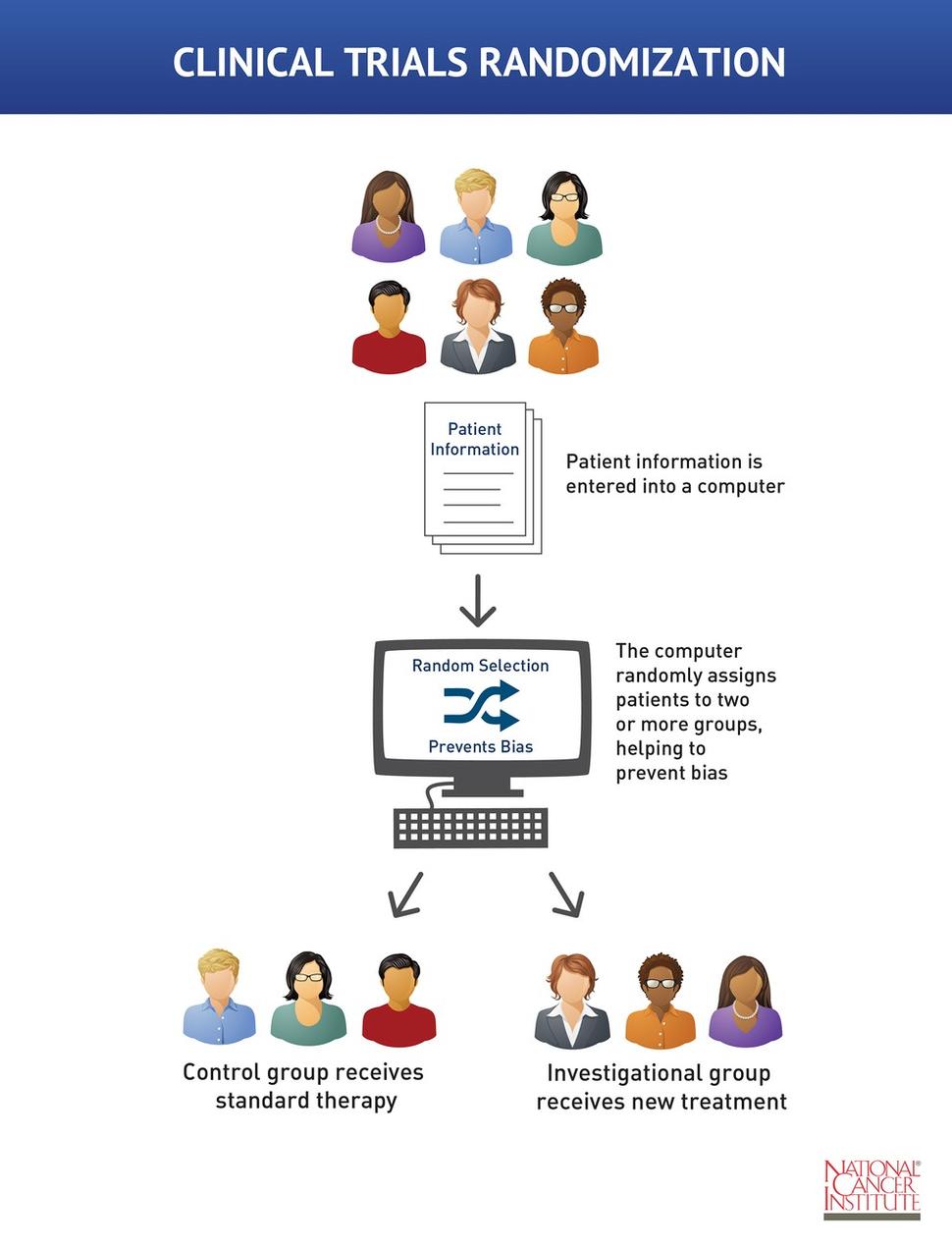
Reducing bias in open-label trials where blinded outcome ... by BC Kahan · 2014 · Cited by 73 — Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators ... Design characteristics, risk of bias, and reporting of ... - The BMJ In trial CA204-009, which was open label, outcome assessors were aware of the intervention received by study participants, and assessment of the progression free survival outcome could have been influenced by knowledge of the intervention received. ... Furthermore, our risk of bias assessments were not blinded to study results because risk of ... External and internal validity of open label or double‚•'blind ... Risk of bias in internal validity in open-label and double-blind trials Internal validity of a clinical trial is influenced by a number of factorsintroducingapotentialforbias,includingselectionbias, subject retention performance bias, detection bias and attrition bias [6]. Patient selection bias Open-label trial - Wikipedia Open-label trial From Wikipedia, the free encyclopedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered.
PDF What Are Open-Label Extension Studies For? factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation. Some Blinding Techniques in Clinical Trials - Blogger The firewall between the sponsor the investigator/clinical research organization can also be implemented in an open label study or single-blind study so that the sponsor is prevented to access the cumulative data for the primary efficacy endpoint for analysis. ... Brennan C Kahan (2014) Reducing bias in open-label trials where blinded outcome ... Open label extension studies and patient selection biases In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%. Conclusions: Future reporting ideals for open label extension studies are recommended to minimize future biases.
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: •
Bias was reduced in an open-label trial through the removal of ... The ratio of the odds ratios was 1.66, indicating that including unconfirmed events in the definition biased the treatment effect upward by 66%. Conclusion: Modifying the outcome definition to exclude subjective elements substantially reduced bias. This may be a useful strategy for reducing bias in trials that cannot blind outcome assessment.
External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias.
Consent to open label extension studies: some ethical issues Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. Investigators are typically reluctant to unblind the patients' assignment at the point of entry into the open label phase, on the grounds that this may introduce ascertainment bias in the main study.
(PDF) What is an open label trial? - ResearchGate Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may...
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
'Off label' use of imaging databases could lead to bias in AI ... Significant advances in artificial intelligence (AI) over the past decade have relied upon extensive training of algorithms using massive, open-source databases. But when such datasets are used "off label" and applied in unintended ways, the results are subject to machine learning bias that compromises the integrity of the AI algorithm, according to a new study by researchers at the ...
Bias for Patient-Reported Outcomes in Open-Label Cancer ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
Exploring open-label bias in patient-reported outcome (PRO) emotional ... We compared PRO emotional domain results between investigational arms of paired open label and double-blind trials of the same drug and disease population. We hypothesized that greater improvement in emotional domain scores would be found in the investigational arms of open label compared to their paired blinded trials, providing evidence of bias.
FDA Guidance: "Design Considerations for Pivotal Clinical ... •Bias and Variance •Study Objectives ... - For non-masked (open label) studies this means before any patient reaches an outcome. 36. Closing Remarks
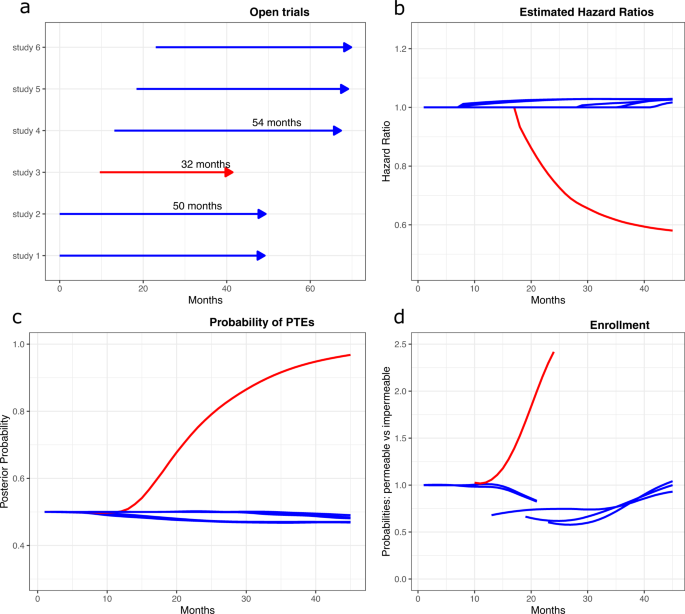
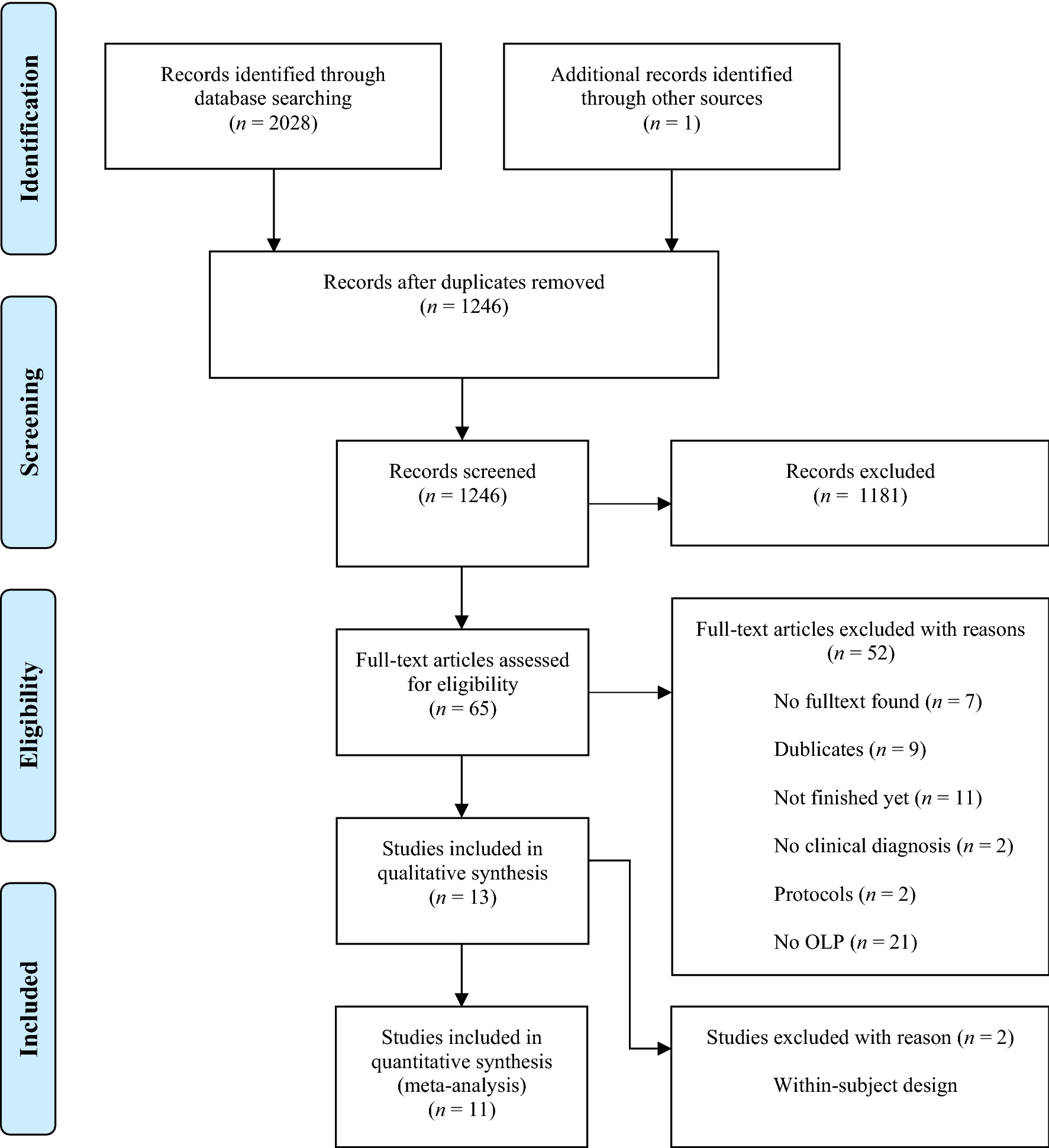
Effects of open-label placebos in clinical trials: a systematic review ... Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the...
Open Label Study | Suggestion Keywords | Top Sites Open-Label Study to Evaluate the Safety, … This is a Phase-1, single center, open label study in approximately 20 adult healthy subjects (Cohort 1 and Cohort 2). Ten subjects will be enrolled in each of the 2 cohorts. The dose of EXPAREL will be determined by the cohort.
A Prospective, Randomized, Open Label Trial of Two Doses of Oral ... None (Open Label) Primary Purpose: Treatment: Official Title: A Prospective, Randomized, Open Label Trial of Two Doses of Oral Betaine in Patients With Non-alcoholic Fatty Liver Disease (NAFLD) and Elevated Alanine Aminotransferase (ALT) Actual Study Start Date : November 12, 2013: Estimated Primary Completion Date : December 30, 2021
PDF Clinical Trial Perspective - Open Access Journals hypothetically) the bias would still be present and would still invalidate the results. The ICH E9 guidance dis tinguishes operational and statistical bias; the former refers to bias introduced during the conduct of the trial, whereas the latter refers to bias introduced in the design, analysis and evaluation of the results. It is also




![PDF] Safety of oral ivermectin during pregnancy: a systematic ...](https://d3i71xaburhd42.cloudfront.net/4e1f7ccf234e029667ee9c730262ec03978ba2bd/5-Table3-1.png)




















Post a Comment for "41 open-label study bias"